Introduction
CD19 CAR-T cell therapy has been revolutionizing the treatment of refractory /relapsed (R/R) B-cell acute lymphoblastic leukemia (B-ALL) over the past decade. While CD7 CAR-T therapy, an innovative approach, has only recently been applied over the last three years in our center to treat R/R T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL). While there are studies and guidelines from EBMT addressing hematologic toxicity, specifically Immune Effector Cell-Associated Hematotoxicity (ICAHT), following CD19 CAR-T treatment, the literature is still scarce on ICAHT occurrence post-CD7 CAR-T therapy. Here we conducted a retrospective study aiming at comparing ICAHT and infection incidences following the two distinct CAR-T therapies.
Methods
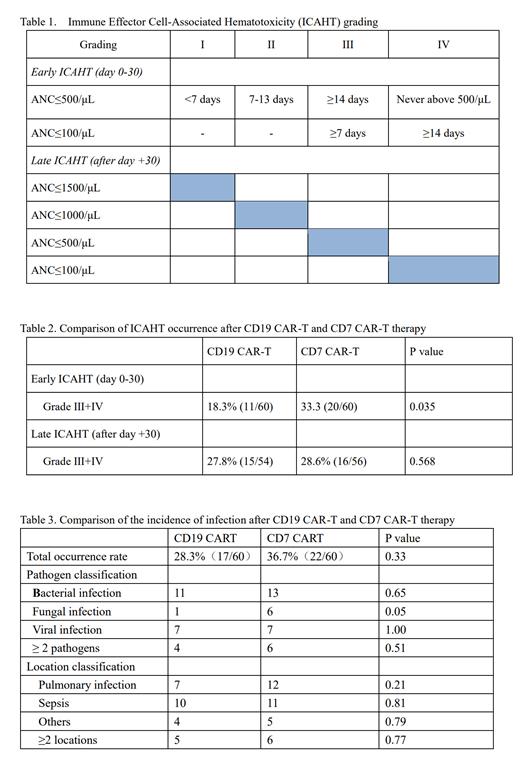
Data were extracted from two separate clinical trials. The CD19 CAR is structured with CD19 scFv, CD28-hinge, 4-1BB costimulatory region, and CD3ζ activation domains. T cells were activated by CD3/CD28 microbead 2 days before CD19-CAR lentivirus infection. “Naturally selected” anti-CD7 CAR-T cells (NS7CAR) were transduced an anti-CD7 CAR into bulk peripheral T lymphocytes via a lentivirus vector. NS7CAR is a 2nd generation murine-based CAR-T containing 4-1BB and CD3ζ co-stimulatory domains. All patients received lymphodepletion regimens of fludarabine (30 mg/m 2/d) and cyclophosphamide (300 mg/m 2/d) from Day -5 to Day -3 before CAR-T infusion. A single infusion of either CD19 CAR-T or CD7 CAR-T cells was administrated at a medium dose of 1×10 6/kg. Fitted patients undergo consolidation allogeneic hematopoietic stem cell transplantation (Allo-HSCT) within 1-3 months after CAR-T cell infusion. The hematology toxicity and infection were analyzed during the period from CD19 CAR-T or CD7 CAR-T cell infusion to Allo-HSCT. Hematology toxicity is graded according to “ICAHT: EHA/EBMT Consumption Grading and Best Practice Recommendations” (Table 1). Infection diagnosis includes both etiological diagnosis and imaging diagnosis.
Results
Between June 1, 2019, and September 22, 2022, 60 patients with R/R B-ALL patients received CD19 CAR-T therapy (https://clinicaltrials.gov NCT04546893, and NCT04792593). Meanwhile, between December 1, 2020, and July 16, 2022, 60 patients with R/R T-ALL/LBL received CD7 CAR-T therapy (NCT04572308, NCT04916860). The CD19 CART group had 19 patients ≤ 14 years old, with a male/female gender ratio of 33:27. The CD7 CART group had 20 patients ≤ 14 years old, with a gender ratio of 49:11.
The incidence of severe early ICAHT (Grade III+IV, day 0-30) from CD19 CAR-T cells infusion to allo-HSCT was observed to be 18.3%, which was lower than the incidence of the early ICAHT post CD7 CAR-T therapy at 33.3%, p =0.035. However, the incidence of severe late ICAHT (after day+30) following CD19 CAR-T and the CD7 CAR-T therapy was similar: 27.8% vs. 28.6%, with no statistical difference( p=0.57).
There was no statistical difference between CD19 CAR-T and CD7 CAR-T therapy groups in the infection rates with 28.3%vs. 36.7%, p=0.33. In the CD19 CAR-T group, bacterial infections were noted in 10 patients: 6 with Gram-positive bacteria (1 Enterococcus, 1 Streptococcus agalactis, and 4 Staphylococcus), and 4 with Gram-negative bacteria (1 Pseudomonas aeruginosa, 2 Acinetobacter baumannii, and 1 Klebsiella pneumoniae). Viral infections were observed in 7 patients (EBV 2, CMV 2, BKV 3), and 1 had fungal infection. Infection sites included pulmonary infections (7 cases), sepsis (10 cases), and other areas (4 cases). Post-CD7-CAR-T, bacterial infections were observed in 9 cases, 7 with Gram-positive bacteria (6 Staphylococcus, and 1 Streptococcus bradycardia) and 2 with Gram-negative bacteria (1 Pseudomonas aeruginosa, and 1 Klebsiella pneumoniae). Six cases had viral infections (EBV 2, CMV 2, HHV1 type 1, parainfluenza virus 1), and 6 with fungal infections. Infection sites included pulmonary infection (12 cases), sepsis (10 cases), and other areas (5 cases).
Conclusions
Our study noted a slightly higher incidence of ICAHT within 30 days following CD7 CAR-T infusion compared to CD19 CAR-T therapy. However, there was no statistical difference in the incidence of ICAHT beyond the 30-day. There was no statistical difference for infection rate after CD19 CAR-T and CD7 CAR-T. In both groups, Gram-positive bacteria were primary responsible for bacterial infections.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal